ARCoV, a new COVID-19 vaccine candidate jointly developed by SIP-based biotech company Abogenbio Suzhou, Academy of Military Medical Sciences, a Beijing-based medical research institute affiliated to Academy of Military Science, and Walvax Biotechnology Co Ltd, a bio-pharmaceutical enterprise in Southwest China’s Yunnan province, was recently approved by National Medical Products Administration of China to proceed to human trials.
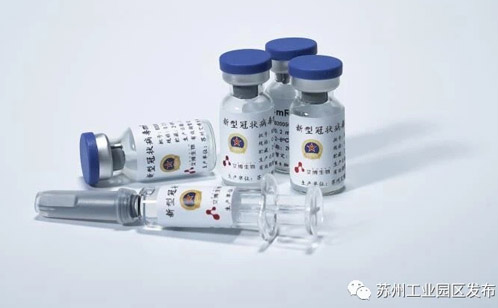
The new drug uses mRNA technology, an approach that is also used for candidates developed in the United States and Germany, but has never been tested in China-based clinical trials.
SIP authorities are giving full support to Abogenbio Suzhou to help push on with the project.







